Atomic Number For Hydrogen
Hydrogen (H) is the first element in the periodic table.
Basic Atomic Number 1 Facts. At room temperature and pressure, hydrogen is a colorless, odorless gas. While ordinarily classified as a nonmetal, the solid form of hydrogen acts like other alkali metals in the same column of the periodic table. The number of atoms or molecules (n) in a mass (m) of a pure material having atomic or molecular weight (M) is easily computed from the following equation using Avogadro's number (NA = 6.022×10 23 atoms or molecules per gram-mole): M mN n A (1) In some situations, the atomic number density (N), which is the concentration of atoms or molecules per. Hydrogen is a chemical element having the atomic number 1 and is given in the symbol H. An atom of hydrogen is composed of one proton and no neutrons in the nucleus; it has one electron in its 1s orbital.
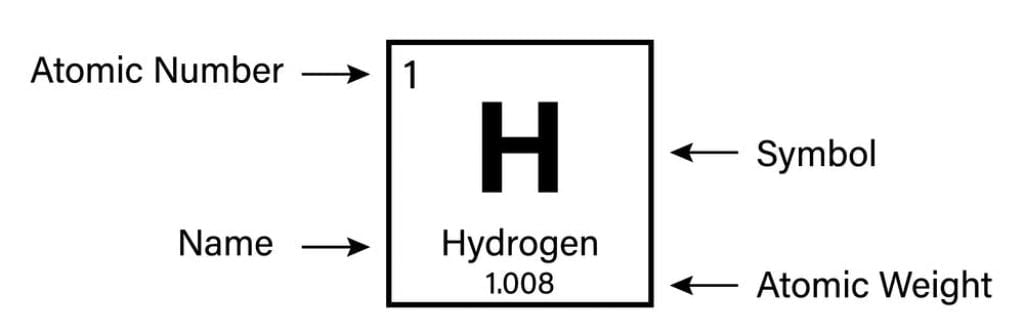
Atomic number, Z = 1
Electronic Configuration : 1s1
Atomic Number For Hydrogen Crossword
Average relative atomic mass: 1.00794 amu
Occurrence
What Is The Atomic Number For Hydrogen? + Example
* Hydrogen is the most abundant element in the universe (92%).
* It also accounts for 15.4% of all atoms in earth’s crust. Super video converter for mac free download.
Atomic Number For Hydrogen 2
* The elemental form is diatomic molecule, H2 and it exists as a gas at room temperature.
Atomic Number For Hydrogen 3
* However the elemental form of hydrogen is highly reactive and hence it occurs in the combined state - mostly as water.

Isotopes of hydrogen
* There are three isotopic forms of hydrogen: Protium (1H1), Deuterium (1H2 or D), Tritium (1H3 or T).
| Isotope | Symbol | No. of Protons (p) | No. of Neutrons (n) | Mass number (A = p + n) | Relative Atomic Mass | Mass % |
| Protium | 1H1 | 1 | 0 | 1 | 1.00782 | 99.985% |
| Deuterium | 1H2 or D | 1 | 1 | 2 | 2.01410 | 0.015% |
| Tritium | 1H3 or T | 1 | 2 | 3 | 3.01604 | 7x10-16 % |
* Protium is the most abundant form of hydrogen. Since it contains only one proton and no neutron, its atomic mass is roughly equal to proton. The percent composition of protium is 99.985%. Hence the average atomic mass of hydrogen is equal to 1.00794 amu.
* Deuterium is called heavy hydrogen. It constitutes 0.015% of total hydrogen. It contains a neutron.
* Tritium has two neutrons. It is radioactive and disintegrates by β-decay. It’s half life is 12.26 years. Free download microsoft office for mac os x 10.7 5.
* Tritium is formed in the nature due to following nuclear transformation.
Atomic Number For Hydrogen Atom
* It is used as tracer in the study of mechanism of chemical reactions.
* It is relatively cheap and safe to work with Trituim in the laboratory. It is non toxic due to emission of low energy β-radiation (no γ-radiation).
* The isotopes of hydrogen show similar chemical properties since they possess same electronic configuration. But they show different physical properties due to different masses.

* However the reactivity of protium is more than that of deuterium. It is due to relatively stronger D-D bond.
www.adichemistry.com
